Water has an extraordinarily high heat of vaporization, which means that turning liquid water into steam requires more energy than most other liquids on Earth. So, why does water have a high heat of vaporization? Let’s take a closer look at the specifics of this intriguing property.
Do You Know What Vaporization Is?
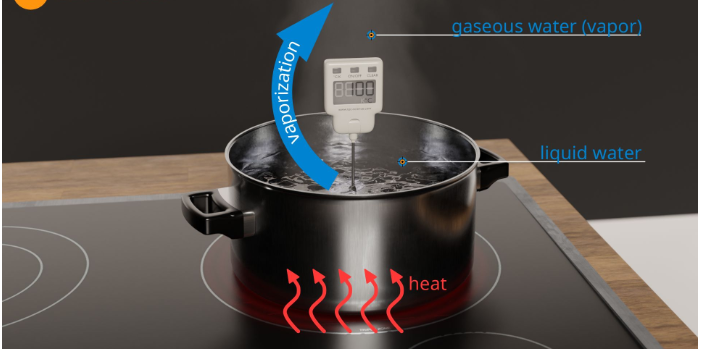
The process through which a liquid state transforms into a vapor state is vaporization. Temperature increases cause molecules’ kinetic energy to increase. The force of attraction between molecules decreases as kinetic energy increases. As a result, they escape in the form of vapors into the environment. In this procedure, heat energy is used.
Vaporization Types
There are two different types of vaporization.
- Evaporation
- Boiling
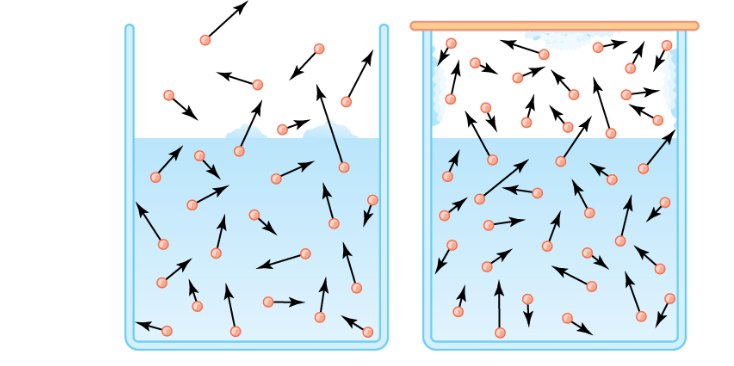
Evaporation differs from boiling because it is a surface phenomenon instead of boiling, which is a bulk phenomenon.
Evaporation is the science behind this medieval practice. Water is kept cool by being stored in earthen containers, especially during the heat. Clay particles make up earthen pots, which have pores in them. When water is poured into the pot, it absorbs heat from the remaining water in the pot and evaporates through the pores.
As a result of the heat loss, the remaining water in the pot cools. As a result, the water is kept chilled, making it suitable for drinking during the summer.
Factors that Influence Vaporization Rate
1 – Temperature
A proportional relationship exists between temperature and vaporization. With increasing temperatures, molecules’ kinetic energy increases. As a result, the attraction force weakens. As a result, as the temperature rises, the evaporation rate rises.
2 – Surface
As the surface area of a particle rises, the rate of vaporization increases as more particles are exposed to temperature changes.
3 – Evaporation and Pressure
Evaporation is inversely proportional. The particles become more difficult to achieve the required kinetic energy and escape as the pressure rises.
4 – Wind speed
As the wind speeds up, the rate rises as the wind carries away particles.
Heat Capacity of Water
Because it must spend some of the heat to break hydrogen bonds between the molecules, increasing the temperature of liquid water takes a lot of energy. Alternatively, water has a high specific heat capacity, which is the amount of heat required to raise the temperature of one gram of a substance by one degree Celsius. The calorie is the amount of heat required to increase the temperature of 1 g of water by 1 °C.
Due to its large heat capacity, water can decrease temperature swings capacity. The specific heat capacity of water is nearly five times that of sand, for example. The sunsets as the land cool faster than the sea, so slow cooling water can leak heat to neighboring land at night. Warm-blooded animals also use water to disperse heat throughout their bodies: it works similarly to a car’s cooling system, transporting heat from warm to cold areas and assisting the body in maintaining an even temperature.
Water Vaporization Heat
Water vaporizes at the highest temperature of any liquid. But why does water have a high heat of vaporization? Because it evaporates (converts to water vapor) at a higher temperature than other liquids, it becomes so hot in the summer, and we sweat! At normal atmospheric pressure and average Earth temperatures, they define the heat of vaporization as the amount of energy necessary to turn one gram of a liquid into one gram of gas (usually 298K). Water has a vaporization heat of 4060 calories per gram, but ethanol has a vaporization heat of 3179 calories per gram.
The enthalpy change when a substance’s solid becomes liquid is measured in the heat of vaporization (or heat of evaporation) per unit mass (gram) or unit volume (cubic centimeter). At the standard boiling point (100 °C), the heat of vaporization for water has been calculated to be 2.26103 J/g. The greater the latent heat of vaporization, the more energy is required to convert it into steam at the specific temperature and pressure.
Important Notes
- It takes a lot of energy to dissociate liquid water molecules, which turns the substance into a gas.
- The boiling point of water is the temperature at which the hydrogen bonds between water molecules are sufficiently broken.
- When the heat of vaporization is reached, water is transformed from a liquid to a gaseous state (steam).
- Sweat (mainly water) evaporates, removing heat from the skin’s surface and cooling the body.
Water heaters consume a significant amount of energy in people’s daily lives. According to the US Department of Energy (DOE), residential water heating consumes 13.1% of the energy delivered to residential structures, according to the US Department of Energy (DOE).
Water Heaters Efficiency
Gas water heaters, such as the Home Tankless Water Heater, account for 36.19 percent of the market, while electric water heaters, such as the Bosch Electric Mini-Tank Water Heater, solar water heaters, and air-source heat pump heaters, respectively, account for 47.36 percent, 8%, and 1%. Water heating accounts for 39% of overall energy consumption, with a gas water heater accounting for 91 percent of total gas usage in a typical American family. As a result, the water heater’s efficiency must be good enough to meet the goals of economics and environmental preservation.

Gas water heaters account for a large portion of the water heater market. However, existing water heaters are either inefficient due to single-use of energy or have a high failure rate due to low-temperature corrosion.
This article proposes a new heat exchanger structure for a gas water heater, with a heat transfer method that includes forced convection and phase change heat transfer, which provides a higher heat transfer coefficient in the same heat exchange area and thus improves the heat exchanger’s efficiency. They conducted an experimental study to examine the differences in efficiency between the new water heater and the current one, and the results revealed that the new water heater is 6 percent more efficient.
This water heater has a moderate temperature shift no matter how rapidly the gas flow increases or decreases. This water heater has a moderate temperature shift no matter how rapidly the gas flow increases or decreases. They completed an economic study to predict the new heat exchanger’s economic efficiency. As a result, the new water heater heat exchanger may greatly improve heat transfer efficiency while lowering failure rates, and it is more cost-effective than the old one.
Conclusion
On a hot summer day, most of us have felt the agony of walking on hot sand or tarmac and the relief of leaping into the cool water of an ocean, lake, or pool to cool off. While water may seem self-evident to provide some respite from heated surfaces, why does it matter? Why does water have a high heat of vaporization? In summary, heating water requires more energy than heating sand or concrete because water has a high heat of vaporization.
Frequently Asked Questions
A: Because the atoms in a pure metal are so close together, they may quickly transfer heat by conduction from one atom to the atoms next to it. As a result, the quantity of energy required to heat metal is comparatively low compared to water.
For example, adding heat does not raise the temperature when water is boiling. It occurs when any substance that may evaporate reaches its boiling point. Adding heat energy to a liquid at boiling point turns it into a gas without raising the temperature.
The addition of energy (heating) causes atoms and molecules to move faster, resulting in a temperature rise. Removing energy from atoms and molecules (cooling) causes their motion to slow down, resulting in a drop in temperature. Conductivity refers to the addition or removal of energy from a substance.
When added to water, fifty-eight grams of dissolved salt per kilogram of water raises the boiling point or the temperature at which the water will boil. Also, the temperature required to boil increases by around 0.5 C. It is a type of boiling point elevation that isn’t limited to water.
